Adipromin Research
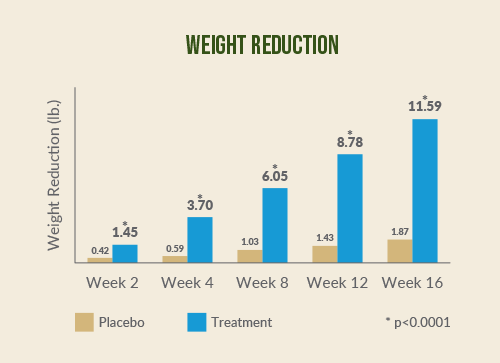
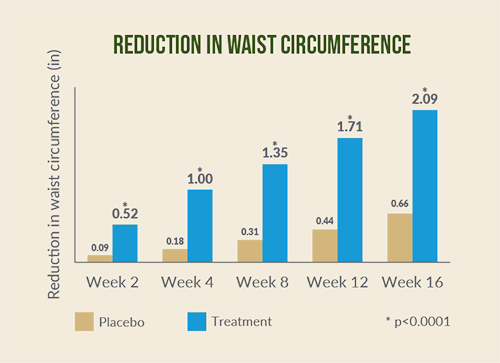
Fig. 1 Reductions in body weight and in waist circumference of Adipromin vs placebo. Dixit K et al. Diabetes Obes Metab. 2018 Nov;20(11):2633‐2641.
Consistent, Steady Results Shown in Both Women and Men
Study Results
The clinical efficacy of Adipromin has been demonstrated in three randomized, double-blind, placebo-controlled clinical trials, published in well-respected medical journals.4, 5, 6 To
validate weight management claims, health professionals and regulatory
agencies alike prefer to see at least two clinical trials published in
the peer-reviewed medical literature.
The second, larger study was a randomized controlled
clinical trial of Adipromin in healthy overweight subjects (n = 140).
The 16-week study compared the use of Adipromin in conjunction with an
1800 calorie/day diet and walking plan to a placebo with the same diet
and walking plan.5
At the end of the study, subjects taking Adipromin showed
significant reductions in body weight (-11.82 pounds vs. -1.92 pounds; p
< 0.0001) and BMI (2.05 kg vs. 0.34 kg/m2; p < 0.0001), compared
with placebo.*
Subjects taking Adipromin had significant reductions in waist
circumference (-2.12 inches vs. -0.67 inches; p < 0.0001) and hip
circumference (-1.77 inches vs. -0.47 inches p < 0.0001) compared
with placebo.*
Subjects taking Adipromin had statistically significant improvement
compared to placebo in their blood lipid profiles (p < 0.0001). *
Subjects taking Adipromin had improved serum adiponectin (p =
0.0071) and serum ghrelin (p = 0.0568) compared to those taking
placebo.*
The third clinical trial was a randomized,
double-blind, placebo-controlled clinical trial that assessed the
effects of 7 days of Adipromin supplementation on resting metabolic rate (RMR), heart rate, blood pressure, and indices of mood in healthy overweight men and women.6 Adipromin
produced significant increases in RMR in healthy overweight men and
women relative to those RMR changes measured in a matched placebo group.* The increase in RMR (up to 15.2% after one week) occurred without concomitant alterations in heart rate or blood pressure and was accompanied by an improved measure of mood.*
*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
^A randomized controlled clinical trial of Adipromin vs. placebo, in conjunction with an 1800-calorie diet and walking plan, was conducted with 140 healthy overweight subjects. After two weeks, subjects taking Adipromin lost 1.47 pounds; those taking placebo lost 0.42 pounds. After 16 weeks, the Adipromin group lost 11.82 pounds while the placebo group lost 1.92 pounds. At the end of the study, those taking Adipromin lost 1.77 inches around the hips compared to 0.47 inches in the placebo group; and they lost 2.12 inches around the waist vs. 0.67 inches in the placebo group. Those taking Adipromin also had statistically significant changes to blood lipids compared to those taking placebo. Adipromin is recommended for use in conjunction with a diet and exercise plan.
‡Compared to diet and exercise alone.
†These mechanisms of action for Adipromin were demonstrated in pre-clinical research, not in humans.
REFERENCES1Sengupta K, Golakoti T, Chirravuri V, Marasetti A. An Herbal Formula LI85008F Inhibits Lipogenesis in 3T3‐L1 Adipocytes.Food Nutr Sci2011;2(8):809‐817.2Kundimi S, Kavungala KC, Sinha S, Tayi VNR, Kundurthi NR, Golakoti T, Davis B, Sengupta K. Combined extracts ofMoringa oleifera,Murraya koeingiileaves, andCurcuma longarhizome increases energy expenditure and controls obesity in high‐fat diet‐fed rats.Lipids Health Dis. 2020 Aug 28;19(1):198..3Krishnaraju AV, Sundararaju D, Srinivas P, Rao CV, Sengupta K, Trimurtulu G. Safety and toxicological evaluation of a novel anti‐obesity formulation LI85008F in animals.Toxicol Mech Methods. 2010 Feb;20(2):59‐68.4Sengupta K, Mishra AT, Rao MK, Sarma KV, Krishnaraju AV, Trimurtulu G. Efficacy and tolerability of a novel herbal formulation for weight management in obese subjects: a randomized double blind placebo controlled clinical study.Lipids Health Dis. 2012 Sep 20;11:122.5Dixit K, Kamath DV, Alluri KV, Davis BA. Efficacy of a novel herbal formulation for weight loss demonstrated in a 16‐week randomized, double‐blind, placebo‐controlled clinical trial with healthy overweight adults.Diabetes Obes Metab. 2018 Nov;20(11):2633‐2641.

Holistic Weight Management - Natural Interventions for Weight Loss

Adipromin
Modern Science Improves on Traditional Healthcare
Adipromin is a patented formulation of three standardized herbal extracts: turmeric (Curcuma longa), moringa (Moringa oleifera) and curry leaf (Murraya koenigeii). It was developed through an extensive process of screening botanicals to identify maximum, safe weight loss potential. All three of these herbs have been in long use in Asian and Indian cuisines. Each is sustainably harvested, non-GMO, and traceable from the fields where they were grown, through processing, all the way to the product you receive.